Search

Free Family-Friendly Science Fun During National Science Week 2025. Get ready for an awesome adventure into the world of Science, Technology, Engineering and Mathematics!

National research led by the Wesfarmers Centre of Vaccines and Infectious Diseases, based at The Kids Research Institute Australia, has secured more than $3.4 million to assess the epidemiology of respiratory syncytial virus (RSV) throughout the country and optimise Australia’s immunisation strategy.

A new study underway at the Wesfarmers Centre of Vaccines and Infectious Diseases, based at The Kids Research Institute Australia, is deliberately infecting tonsils with Strep A in the laboratory to test a range of potential vaccine candidates.
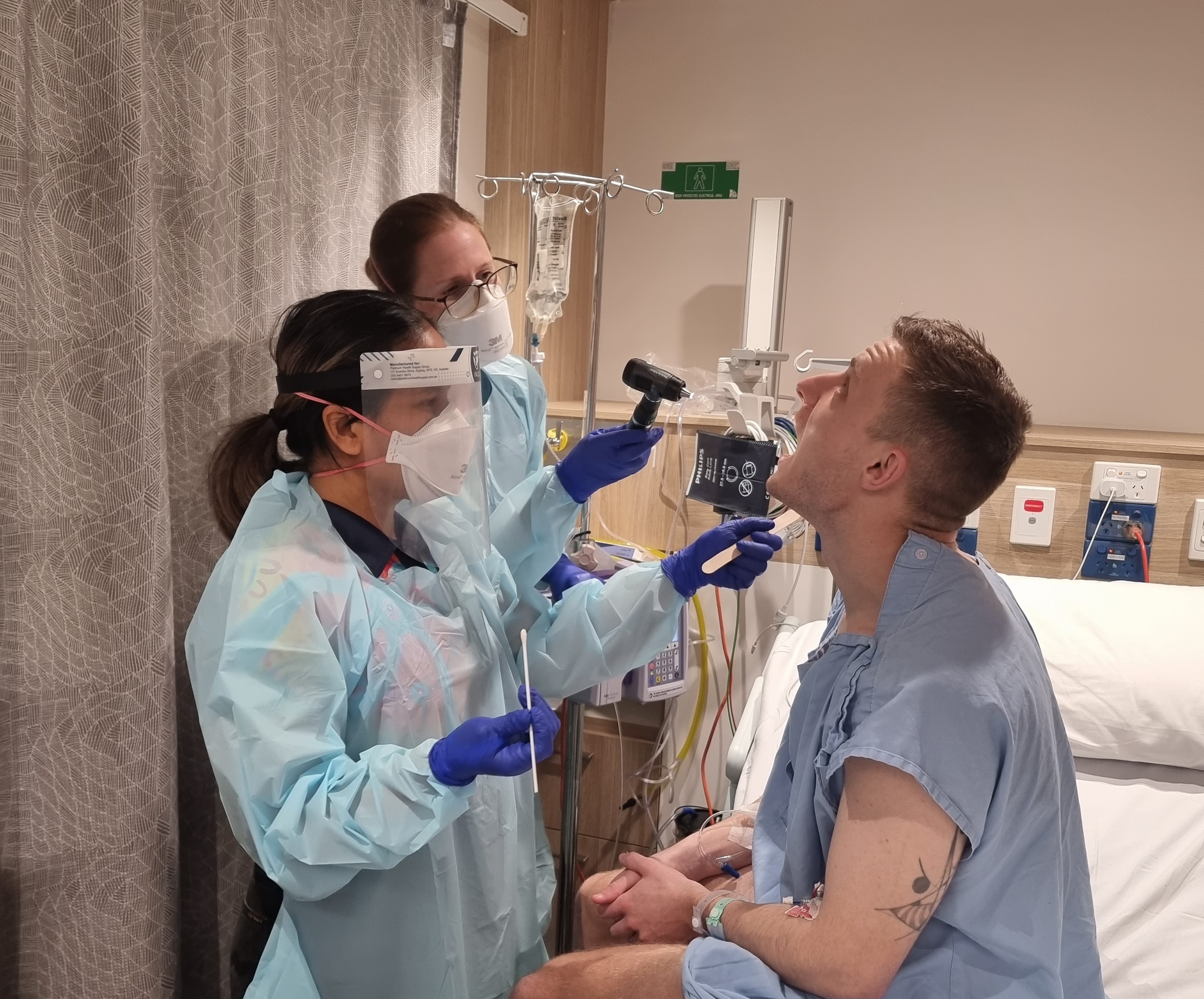
A unique study purposely giving participants Streptococcus pyogenes (Strep A) to learn how much penicillin it takes to prevent infection has found the amount needed is much lower than previously thought – a discovery that will transform thinking on treatment for people living with rheumatic heart disease (RHD).

Two infectious disease researchers from Papua New Guinea (PNG) dedicated to reducing rates of childhood mortality in their home country are making significant advances thanks to support from the Deborah Lehmann Research Award (DLRA).

A life-saving meningococcal vaccine covering all five common strains of the deadly disease could soon be available thanks to vital research demonstrating the safety and effectiveness of a combination Men ABCWY vaccine.

Researchers from the Wesfarmers Centre of Vaccines and Infectious Diseases, based at The Kids Research Institute Australia, have launched an online guidance tool designed to help families and health-care providers in WA learn the best way to protect babies and young children against life-threatening respiratory syncytial virus (RSV).

Two projects led by The Kids Research Institute Australia have been awarded more than $2.5 million to fund innovative ideas focused, respectively, on combating persistent ear infections and investigating how dangerous fungi invade the bodies of immunocompromised people.

We honour the memory of Emeritus Professor Michael Alpers, a colleague and friend to many at The Kids Research Institute Australia, who passed away on December 3, 2024.

Congratulations to six researchers from The Kids Research Institute Australia, who will use valuable support from the Raine Medical Research Foundation’s 2024 grant round to undertake projects focused on improving the health and wellbeing of babies, children and young people.
